Magnesium oxide has good chemical inertness, heat resistance, insulation and thermal conductivity. The most prominent ones are good resistance to high temperature oxidation, moderate alkalinity, electrophilicity due to the presence of oxygen vacancies and single electrons, etc. , these properties provide important prerequisites and foundations for the application of magnesium oxide. One-dimensional or two-dimensional MgO structures, such as nanorods, nanoribbons, and nanowires, have successfully explored a variety of experimental synthesis methods.
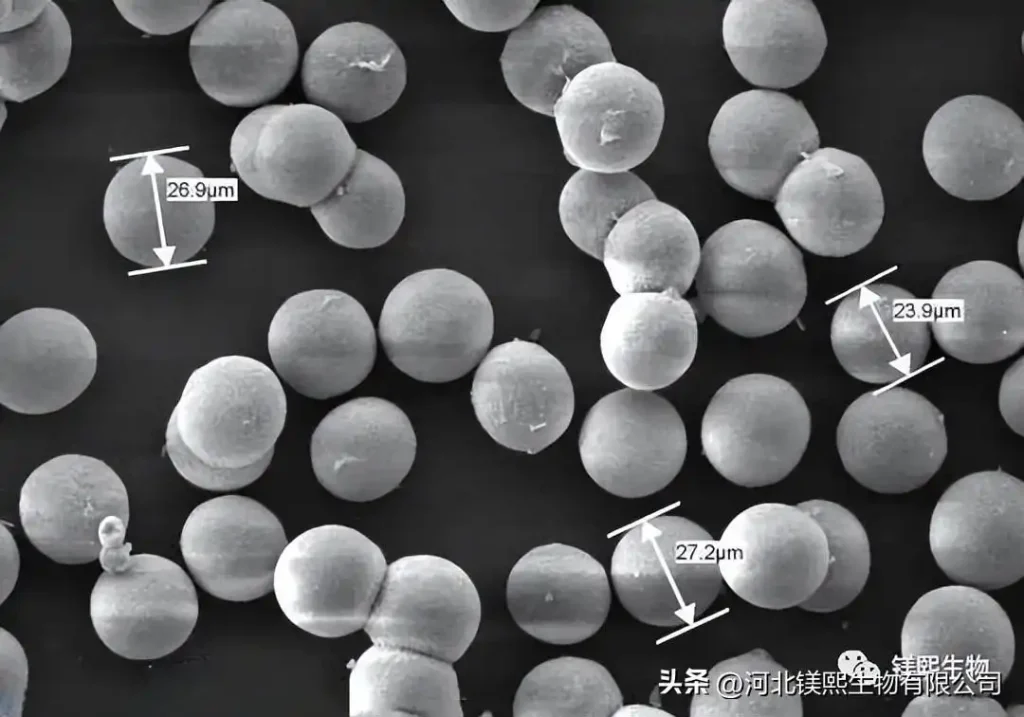
However, there are few reports on the preparation method of three-dimensional spherical MgO. After research, it has been found that some special morphology of magnesium oxide has very effective applications in many aspects.
Application of spherical magnesium oxide
Spherical magnesium oxide is mainly used in chromatography stationary phase, adsorption of toxic substances, and as material additives.
1. Chromatography stationary phase
The effective separation effect of high performance liquid chromatography is closely related to the packaging material used in chromatography, that is, the stationary phase. The current packaging materials for liquid chromatography are mainly silica and packaging materials based on it, but it also has problems such as poor stability, reactions with some basic substances, and difficulty in separation.
Mixing magnesium and aluminum oxides with silica in a certain proportion as the stationary phase of liquid chromatography can solve this problem well. Compared with traditional silicon as a stationary phase, spherical magnesium oxide has high selectivity and high efficiency in the separation of basic chemicals, Therefore, the application of spherical magnesium oxide in liquid chromatography has received more and more attention.
2. Spherical magnesium oxide adsorbs pollutants in water
Mesoporous nanosheets of spherical magnesium oxide exhibit excellent adsorption properties and can adsorb common toxic heavy metal ions and organic pollutants, and are promising for use in wastewater treatment processes.
3. Spherical magnesium oxide as an additive for polymer materials
General plastics have very low flame retardancy and are prone to accidents. For example, in LED lamps, if used for a long time, the temperature inside the lamp will be very high. If the heat cannot be released in time, accidents may easily occur. Generally, some inorganic substances are added to plastics to improve the thermal conductivity of plastics. Common flame retardants include zinc borate, aluminum oxide and magnesium oxide.
Generally speaking, alumina and magnesium oxide are relatively easy to obtain, but the preparation process of spherical magnesium oxide is not yet mature, so now alumina is a widely used flame retardant additive. However, the high performance flame retardancy of spherical magnesium oxide makes the industry very optimistic about its prospects.
Preparation method of spherical magnesium oxide
Magnesium oxide with a spherical structure is calcined from the precursor spherical basic magnesium carbonate or spherical magnesium hydroxide or spherical basic magnesium oxalate. Taking spherical basic magnesium carbonate as an example, the spherical structure of basic magnesium carbonate is formed by stacking basic magnesium carbonate with a flaky structure. When basic magnesium carbonate is calcined, the spherical basic magnesium carbonate undergoes thermal decomposition to obtain spherical magnesium carbonate. Magnesium oxide. The synthesis methods of spherical basic magnesium carbonate can be divided into two types: direct synthesis and indirect synthesis.
1. Direct synthesis method
The direct synthesis method is a widely used synthesis method in the preparation of spherical basic magnesium carbonate. This method is directly synthesized at a specific reaction temperature without changing any experimental conditions, and directly obtains spherical basic magnesium carbonate.
(1) SDS-assisted low-temperature synthesis method
Using magnesium chloride and sodium carbonate as experimental raw materials, with the assistance of sodium lauryl sulfate, spherical basic magnesium carbonate was directly synthesized at a temperature below 55°C. Although the synthesis temperature is low, the reaction time is as long as 24 hours, which has certain limitations in terms of time cost.
(2) Additive-assisted synthesis method at low temperature
At a certain reaction temperature, different additives, such as sodium tartrate, polyacrylamide, and manganese chloride, are evenly dissolved in the stirring deionized water. While stirring, the magnesium chloride solution and sodium carbonate solution are mixed simultaneously. Add dropwise to the reactor, continue stirring for a specific period of time, then stop and let it stand for 4 days to obtain spherical basic magnesium carbonate.
(3) Direct synthesis by co-precipitation method
Using soluble magnesium salts and soluble carbonates as experimental raw materials, the pH value in the aqueous solution system was adjusted with hydrochloric acid or ammonia, and a hydrothermal synthesis reaction was used to prepare nearly spherical bird’s nest-shaped basic magnesium carbonate.
2. Indirect synthesis method
The indirect synthesis method is a synthesis method that first synthesizes other forms of magnesium carbonate, and then generates spherical basic magnesium carbonate after pyrolysis.
(1) Method of adding sodium oxalate to heavy magnesium water
Lightly burned dolomite powder was used as the experimental raw material, and the carbonization method was used. Sodium oxalate was added to remove calcium, and then heavy magnesium was hydropyrolyzed to prepare a high-purity magnesium oxide sample. Then pyrolysis is performed, and the pyrolysis product is basic magnesium carbonate. The basic magnesium carbonate is calcined to obtain a spherical magnesium oxide product.
(2) Carbon dioxide regulation method
Magnesium sulfate heptahydrate and sodium carbonate are used as reaction raw materials. At the reaction temperature, the sodium carbonate solution is added to the reaction system at a constant speed while the magnesium sulfate solution is stirred. After completion, carbon dioxide gas is introduced into the reaction system to adjust the crystal form of magnesium carbonate. This process is a carbonization process. Then let it sit for a while, filter and wash. The obtained product is subjected to a pyrolysis reaction to completely convert the product into basic magnesium carbonate.
write at the end
Magnesium oxide has good chemical inertness, heat resistance, insulation and thermal conductivity. For specific application backgrounds and applications, the morphology and surface properties of magnesium oxide can be adjusted to control its physical morphology, surface area, pore size and The pore volume, organically combining the chemical properties of magnesium oxide with its morphology and surface properties, will provide a broader space for the application of magnesium oxide with special morphologies.
At present, the preparation process of spherical magnesium oxide is not yet mature, and its excellent properties have not been recognized by most people. Therefore, the development of the preparation process of spherical magnesium oxide and its wider application have good market prospects.
