Hebei Messi Biology Co., Ltd. stated that as an important functional inorganic material, magnesium oxide is widely used in the fields of metallurgy, electronics, catalysis, ceramics, coatings, medicine, adsorption, refractory materials and optical materials. Mineral direct pyrolysis, chemical precipitation, gas phase hydration, carbonization, ammonium bicarbonate, spray pyrolysis, hydrothermal, sol-gel and other methods are used for the preparation of magnesium oxide. However, there are still many problems that restrict the development and application of high value-added magnesium oxide, such as long process flow, environmental pollution, irregular product morphology, wide particle size distribution, and serious particle agglomeration.
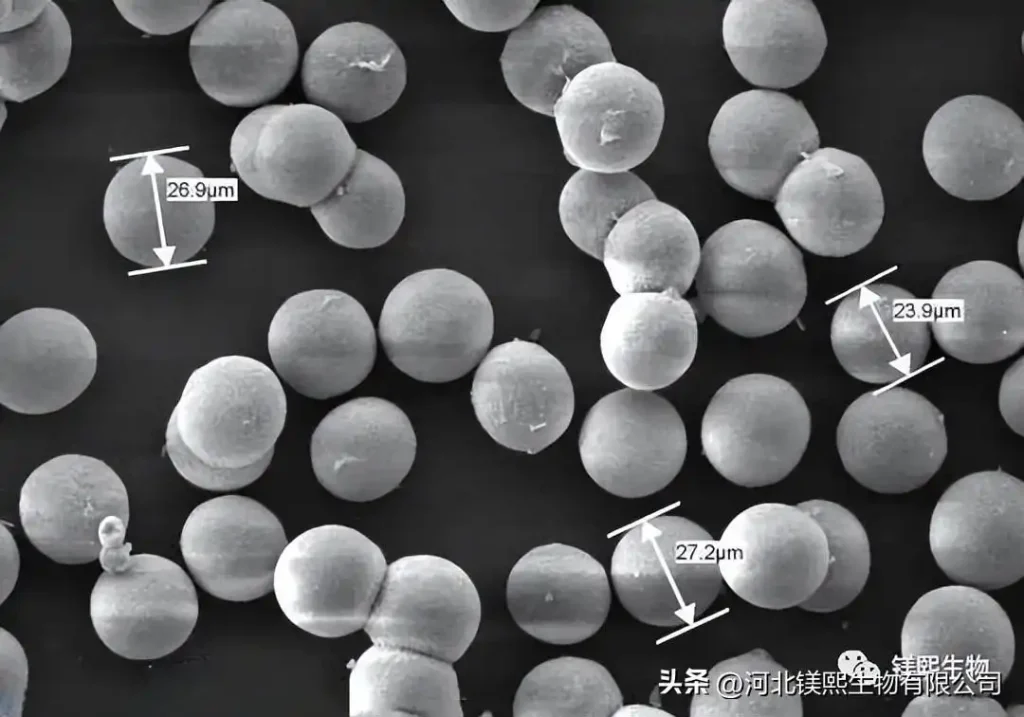
Hebei Messi Biology Co., Ltd. stated that magnesium oxide powders with different morphologies can be prepared by preparing special morphological precursors such as rod-shaped magnesium carbonate, flaky magnesium hydroxide, and massive basic magnesium carbonate, and then performing pyrolysis or calcination. . Among them, spherical magnesium oxide has the characteristics of high reactivity and good stability, and has shown broad application prospects in the fields of catalysts, bactericides, thermally conductive fillers, wastewater treatment, and pollutant adsorption. The preparation of spherical magnesium oxide is mostly obtained by calcining spherical basic magnesium carbonate, spherical basic magnesium oxalate, and spherical magnesium hydroxide as precursors. During the preparation process, it is necessary to use alkaline precipitants and surfaces such as sodium polyphosphate and polyacrylamide. Active agent, and obtain bird’s nest and spherical magnesium oxide structures through growth regulation of sheet structure. At present, there is still a lack of simple preparation method of spherical magnesium oxide. The preparation process and device development of spherical magnesium oxide are of great significance for improving resource utilization and developing new functional materials.
A method for preparing spherical magnesium oxide. The steps are as follows: mix a magnesium salt solution and an auxiliary solution, and configure it into a precursor solution or sol under stirring conditions; Gas the precursor solution or sol through the reaction device, and collect the solid powder to obtain the primary product of magnesium oxide, and recover the by-product; calcine the collected primary product of magnesium oxide at a temperature of 600 to 850°C for 1 to 4 hours to obtain a spherical magnesium oxide product. .
