Hebei Messi Biology Co., Ltd. stated that the technical route for preparing magnesium oxide with special morphology can be roughly divided into two situations: (1) Mainly using magnesium salt as raw material to first obtain the precursor for preparing magnesium oxide with special morphology, and then strictly control the heat treatment Conditions so that the special morphology is retained after heat treatment, thereby obtaining magnesium oxide with special morphology; (2) Preparation through mechanical molding, template method, special chemical synthesis method, etc. In the first case, it is easy to prepare more magnesium oxide with special morphology, and the morphology of magnesium oxide is relatively easy to control. In order to prepare precursors with special morphology, additives are generally added to control the crystallization or precipitation process of the precursor, but the amount of additives is generally not large. In general, using the method involved in the first case, the preparation process is easy to flexibly control, and the preparation cost is relatively low; while the method involved in the second case is often suitable for special situations, and the preparation cost is relatively high.
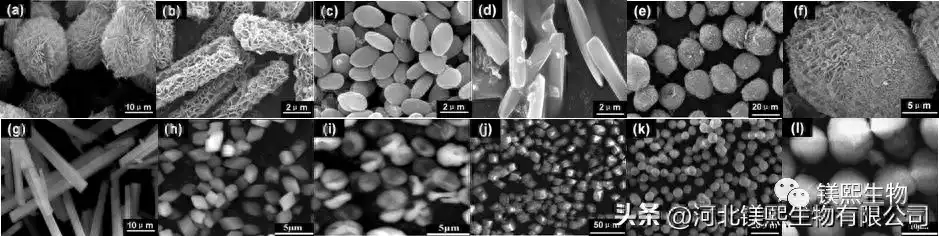
Using the method involved in the first case (precursor method), there may be greater room for development in preparing magnesium oxide with special morphology. The key to using this method is to first prepare a precursor with special morphology. An important way to prepare magnesium salt precursors with special morphologies is to add additives to control the crystallization or precipitation process. At present, there are few research reports on the behavior and mechanism of additives controlling the crystallization and precipitation of magnesium salts, but there are many research works on carbonates for reference. Studying the effects and mechanisms of additives on the crystallization and precipitation behavior of magnesium salts can guide the design, preparation and regulation of precursors, which will provide an important foundation and development space for the preparation of magnesium oxide with special morphology.
Hebei Messi Biology Co., Ltd. stated that magnesium oxide has good chemical inertness, heat resistance, insulation and thermal conductivity. The most outstanding ones are good resistance to high temperature oxidation and moderate alkalinity. Due to the presence of oxygen vacancies and single Electrophilicity generated by electrons, etc. These properties provide important prerequisites and foundations for the application of magnesium oxide. For specific application backgrounds and applications, adjusting the morphology and surface properties of magnesium oxide, controlling its physical morphology, surface area, pore diameter and pore volume, and organically combining the chemical properties of magnesium oxide with its morphology and surface properties will provide The application of magnesium oxide with special morphology provides a wider space.
