Hebei Messi Biology Co., Ltd. stated that the production of magnesium oxide by dolomite carbonization method generally adopts normal pressure and low temperature (≤25%) carbonization method to separate calcium and magnesium to produce light magnesium carbonate, and then calcine to produce light magnesium oxide. The temperature changes greatly in the whole process and the energy consumption is high.
The principle of dolomite carbonization to produce light magnesia is to firstly calcinate dolomite to obtain light-burned dolomite, then digest the light-burned dolomite, remove slag, and send it to the carbonization tower to react with CO from the calciner kiln. During the carbonization process, the carbonate group dissolved in the aqueous solution determines the concentration of the carbonization solution according to the dissociation ratio relationship of the secondary dissociation (ie HCO and CO).
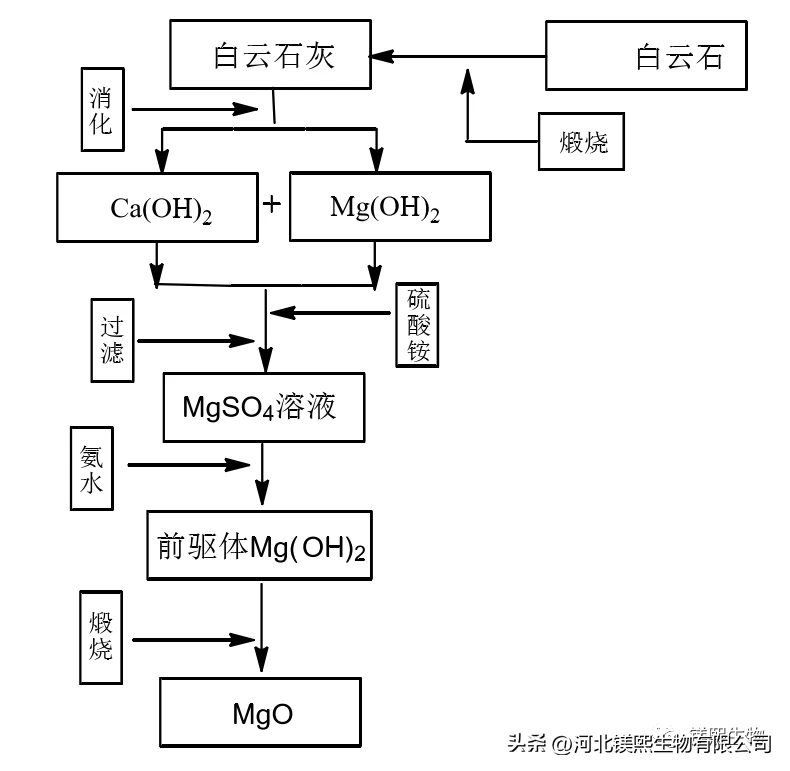
Because calcium hydroxide has strong alkalinity and high activity, it is easy to form calcium carbonate precipitation. When the calcium hydroxide is almost completely converted into calcium carbonate, the pH value of the suspension suddenly drops to 8.5-9, which is determined by the balance of the magnesium hydroxide solution. At 25°C and a pH value of 8.5, 97.5% of the carbonate radical dissociates in the form of HCO, and magnesium hydroxide continues to dissolve into the liquid phase to generate magnesium bicarbonate. Anti-dissolution of calcium carbonate. Magnesium bicarbonate solution (commonly known as heavy magnesium water) is obtained after filtration, and finally heavy magnesium hydrolysis is pyrolyzed to obtain basic magnesium carbonate, which is filtered again and dried to obtain basic magnesium carbonate. Magnesium oxide can be obtained by calcining basic magnesium carbonate at 700°C to 1000°C.

Can you supply full line to produce ccm 5MT / hr from dolomite
Can you supply full line to produce 5MT/hr of ccm from dolomite