Hydrotalcite is a layered columnar double metal hydroxide, which is a kind of anionic clay that has developed rapidly in recent years. Hydrotalcite has special structure and physical and chemical properties, such as basicity, charging properties, anion exchangeability, microporous structure, thermal stability, memory effect, adsorption performance, catalytic performance, etc. Combustion agent, heat stabilizer, catalyst, catalyst carrier, insecticide, sewage treatment agent, electrorheological regulator, medicine, medicine carrier and petroleum industry have a wide range of applications.
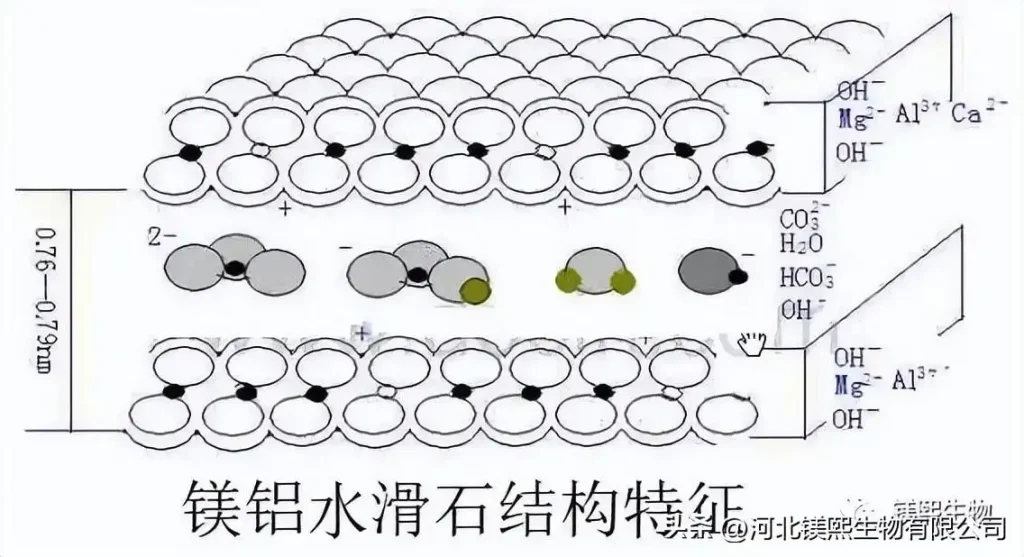
Magnesium aluminum hydrotalcite is a new type of multi-effect plastic additive, which has the similar structure and composition of magnesium hydroxide and aluminum hydroxide. It can absorb heat when decomposed by heat, and can reduce the temperature of the combustion system; it dilutes flammable gases and isolates oxygen at the same time. , to play a flame retardant effect, is a promising new high-efficiency, non-toxic, low-smoke halogen-free flame retardant, so it has great potential for development. The main elements are magnesium and aluminum. Magnesium hydroxide is the basic raw material for the synthesis of hydrotalcite, and a large amount of magnesium element required for the synthesis of hydrotalcite is provided by magnesium hydroxide. The main synthesis methods include co-precipitation method, hydrothermal method, roasting recovery method, template synthesis method, nucleation/crystallization isolation method, etc.
As a flame retardant, hydrotalcite has both the advantages of aluminum hydroxide and magnesium hydroxide flame retardants, and overcomes their respective shortcomings. At present, the main flame retardant materials used in the electrical industry are granular magnesium hydroxide and aluminum hydroxide . In terms of rising material temperature, reducing the heat release on the surface of the material, increasing the natural temperature of the material, and prolonging the ignition time, the effect of aluminum hydroxide is better than that of magnesium hydroxide. In terms of increasing the spontaneous combustion temperature of the material, increasing the oxygen index, and promoting the carbonization effect, magnesium hydroxide is superior to aluminum hydroxide.
Experiments have shown that when the hydrotalcite added to the polymer is decomposed by heat, the released inert gas carbon dioxide and water vapor can dilute the concentration of combustible gases and weaken the fire, and decompose to obtain magnesia and alumina. It also absorbs heat and reduces the surface temperature of the polymer. It can be seen that hydrotalcite does have three functions of flame retardancy, smoke elimination and filling, and is a new type of inorganic flame retardant. At the same time, high-purity smoke suppressant is added, which effectively reduces the amount of smoke generated when the material is burned.
