As a common hydrated magnesium-rich carbonate mineral, hydromagnesite [Mg5(CO3)4(OH)2·4H2O] can be used as a high-quality mineral-type flame-retardant filler in the field of polymer flame-retardant. Studies have shown that hydromagnesite mainly occurs in carbonate-type saline lakes and Quaternary lacustrine strata, and was also found in the lacustrine deposits of Jezero Crater on Mars, and the Mg/Ca value of the lake water is considered to be hydromagnesite. Key conditions for mineralization. The traditional view is that the formation of lacustrine hydromagnesite is mainly related to the weathering of ultrabasic rocks to provide magnesium-rich source supply, but the Mg/Ca value of surface river water and groundwater related to the weathering of ultrabasic rocks rarely reaches enough Threshold for direct precipitation of hydromagnesite in salt lakes (Fig. 1a). Therefore, finding out the geochemical cycle process of Mg in the saline lake system is the key link to understand the mineralization process of hydromagnesite.
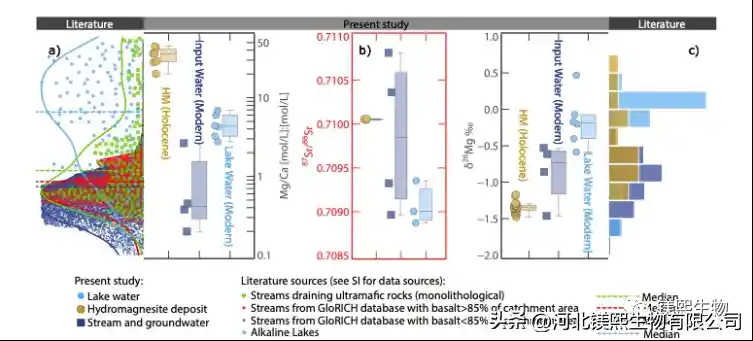
Fig.1 Chemical composition of Dujiali Salt Lake water, recharge water and hydromagnesite: (a) Mg/Ca value (edge density map shows global river water and carbonate-type saline lake); (b) Sr isotope value; ( c) Mg isotope values (marginal histograms are the reported δ26Mg values of hydromagnesite)
In response to the above key scientific issues, relying on the Qinghai-Tibet Plateau Salt Lake Field Scientific Observation and Research Station of the Ministry of Natural Resources, the Institute of Mineral Resources of the China Geological Survey (hereinafter referred to as the “Resource Institute”) and its collaborators took the Dujiali Salt Lake in Tibet as an example to systematically carry out Mg isotopic studies of salt lake water, river water, groundwater, and hydromagnesite (Fig. 1c). In this work, using Mg isotope analysis combined with the hydrochemical simulation software Phreeqc, the semi-quantitative hydrochemical evolution model of the Mg/Ca value and δ26Mg changes in the Dujiali Salt Lake under evaporation conditions was established for the first time (Fig. geochemical cycles.
The results show that under strong evaporation conditions, aragonite first reached saturation and precipitated during the evolution of Dujiali Lake, which led to an increase in the Mg/Ca value and alkalinity of the lake water, thereby driving hydromagnesite to reach saturation and precipitate. It provides a good parameter limit for the model. In the vast majority of lacustrine hydromagnesite deposits in the world, hydromagnesite is usually symbiotic or alternately deposited with aragonite, further confirming the model results. In summary, this work suggests that although surface river water or groundwater associated with the weathering of Mg-rich rocks provides an important source of Mg for the salt lake, lithic precipitation under evaporative conditions may be an important factor driving the deposition of hydromagnesite in the salt lake. mineralization process.
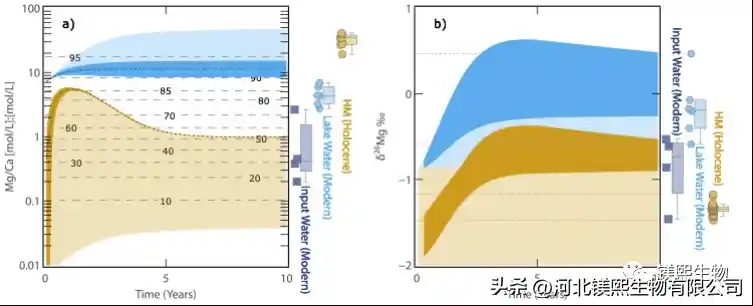
Fig. 2 Results of hydrochemical evolution model of Dujiali Salt Lake (a) Mg/Ca value of lake water (blue) and hydromagnesite (brown); (b) lake water (blue) and hydromagnesite (brown) δ26Mg value.
