Cobalt is an extremely important strategic metal, mainly used in cemented carbide, ceramic color glaze, battery material, superalloy and magnetic material and other fields. In recent years, as the country vigorously develops the new energy industry, the cobalt smelting industry has developed vigorously, and the market demand for cobalt raw materials has become increasingly strong.
Africa\’s Congo (Kinshasa) is rich in copper and cobalt resources, and its cobalt reserves account for nearly 50% of the world\’s total reserves. In recent years, more and more cobalt smelting and production enterprises in my country have built local factories in the Democratic Republic of the Congo to process and produce crude cobalt salts in order to reduce production costs and raw material transportation costs. It has gradually become an industry trend to replace the import of cobalt concentrate with crude cobalt salt.
The currently commonly used crude cobalt salt production process has a series of shortcomings such as unsatisfactory impurity removal effect, high consumption of auxiliary materials, and low cobalt grade of the product. It is urgent to develop a new process or optimize the existing crude cobalt salt production process to reduce production. cost, improve product quality and enhance product competitiveness.
In this paper, a cobalt-containing and low-copper raffinate in Congo (Kinshasa) was used as raw material, and high-grade crude cobalt hydroxide was prepared through the process of impurity removal-first-stage cobalt precipitation-second-stage cobalt precipitation-filtration washing-flash drying. Different from the usual process of returning the second-stage cobalt underflow to the impurity removal or leaching process to ensure product quality, this paper returns all the second-stage cobalt underflow to the first-stage cobalt as reaction seeds, thereby greatly improving the recovery rate of cobalt and increasing the crude oil. The output and comprehensive recovery rate of cobalt hydroxide products, and its cobalt content is over 39%.
1 Raw material equipment and test method
1.1 Raw materials for production
The cobalt-containing raffinate used in the production is a low-copper cobalt sulfate solution obtained from a copper-cobalt ore in the Democratic Republic of the Congo after grinding, leaching, and extraction. The main chemical components are shown in Table 1.

Table 1 Main chemical components of cobalt-containing and low-copper raffinate/(g L-1)
1.2 Main production accessories
Quicklime: Industrial grade, -74μm particle size accounted for not less than 80%, active calcium oxide main content not less than 75%.
Sodium metabisulfite: industrial grade, -74μm particle size accounts for not less than 70%, and the main content is not less than 93%.
Magnesium Oxide: Industrial grade, -74μm particle size accounts for not less than 90%, the main content is not less than 95%, and the activity is not less than 85%.
Compressed air: industrial grade, the air pressure is not lower than 0.4MPa.
1.3 Sample analysis instruments and equipment
X-ray fluorescence spectrometer, flame atomic absorption spectrophotometer, inductively coupled plasma spectrometer, potentiometric titrator, electronic balance, digital display pH meter and acid-base titration platform, etc.
1.4 Test principle
The treatment process mainly consists of processes such as impurity removal and cobalt precipitation. Impurity removal is by adding a certain proportion of sodium pyrosulfite slurry and compressed air to the low-copper and cobalt raffinate to adjust the reaction redox environment, and oxidize the Fe2+ that is difficult to hydrolyze and precipitate into Fe3+ that is easier to hydrolyze and precipitate, and at the same time pass through the quicklime slurry The pH value of the solution is adjusted by the material to achieve the purpose of removing most of the iron ions. In addition, impurity ions such as Cu and Mn can also be partially removed during the impurity removal process. Precipitation of cobalt is mainly done by adding active magnesium oxide slurry, controlling the pH value of the reaction, so that the Co ions in the solution are precipitated as Co(OH)2 precipitates, and then through the process of filter press slurry washing, flash drying and other processes to obtain a water content of about 8 % cobalt hydroxide dry product.
1.4.1 Removal of impurities
The relevant chemical reactions involved in the impurity removal reaction are as follows:
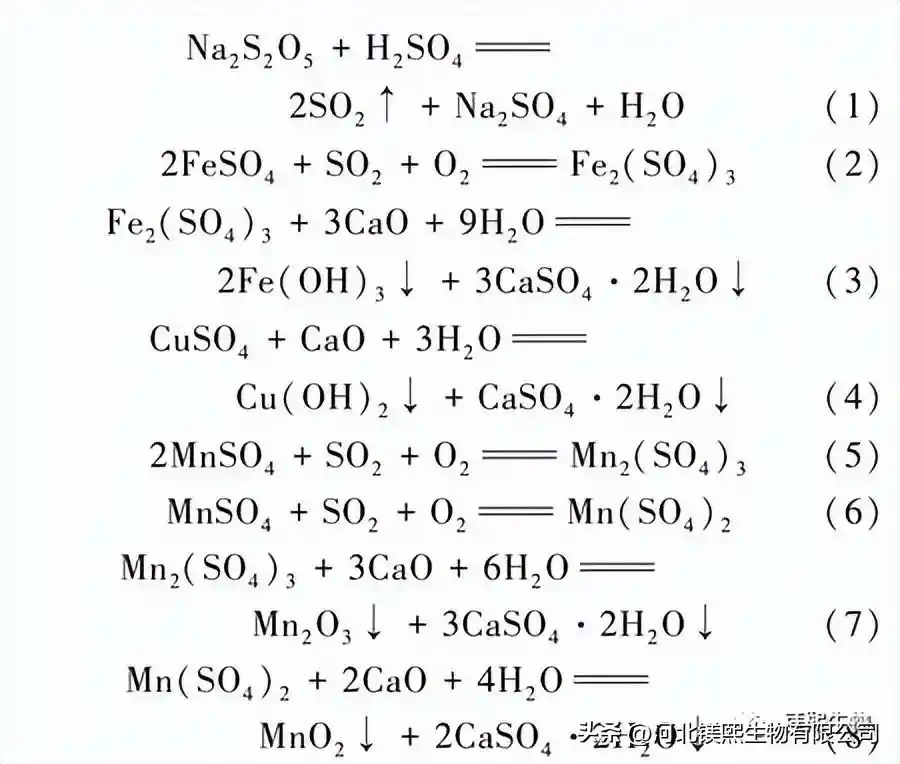
1.4.2 Cobalt precipitation reaction
The relevant chemical reactions involved in cobalt deposition are as follows:
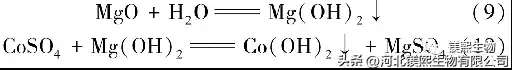
2 Test results and discussion
2.1 Impurity removal process conditions
2.1.1 Effect of limestone slurry concentration on impurity removal effect
Set the impurity removal time to 5h, pH = 4.0, potential 420mV, and the influence of quicklime preparation concentration on the impurity removal effect and cobalt ion loss rate are shown in Figures 1 and 2 respectively. It can be seen from Figure 1 that the preparation concentration of quicklime has little effect on the removal effect of impurity ions. As the concentration increases, the removal rate of iron ions is stable above 99%, and the removal rate of manganese ions basically fluctuates between 35% and 42%. The copper ion removal rate fluctuates between 50% and 60%.
It can be seen from Figure 2 that as the concentration of quicklime increases, the loss rate of cobalt for removal of impurities also gradually increases. When the concentration reaches 30%, the loss rate of cobalt reaches 12.6%. The more quicklime auxiliary materials added to the space, the more easily the impurity removal reaction will be partially over-alkaline, and a large amount of cobalt ions will precipitate out instantly, and the cobalt hydroxide precipitate will be wrapped by calcium iron slag and cannot be returned to dissolve and remove impurities solution, resulting in the loss of cobalt ions during the impurity removal process. The main ions are lost in the impurity removal process, so a lower concentration of quicklime should be selected. However, the lower quicklime preparation concentration will bring greater challenges to the system water balance and the processing capacity of auxiliary material conveying equipment. Considering comprehensively, it is advisable to choose 15% quicklime preparation concentration.
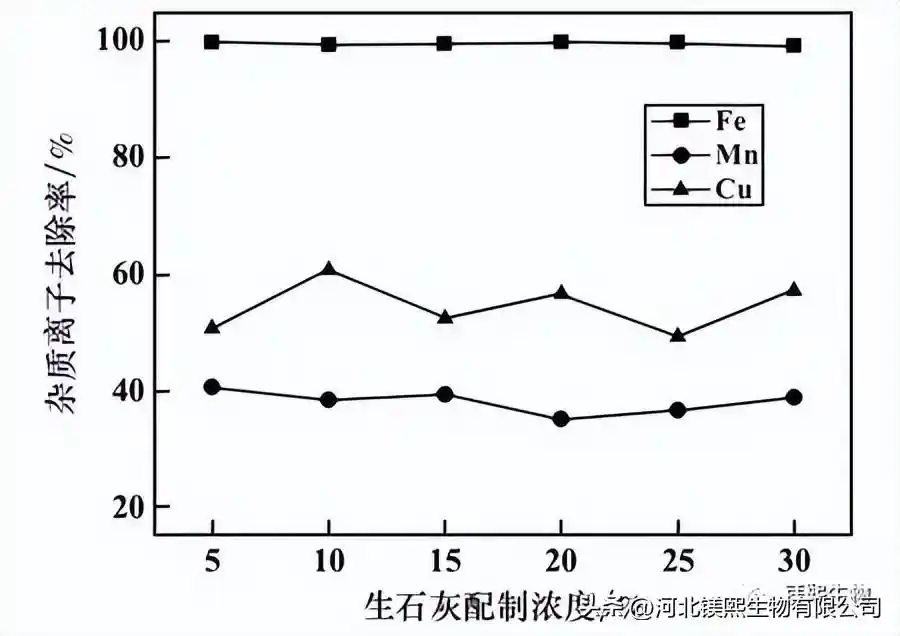
Figure 1 The influence of quicklime preparation concentration on the impurity removal effect
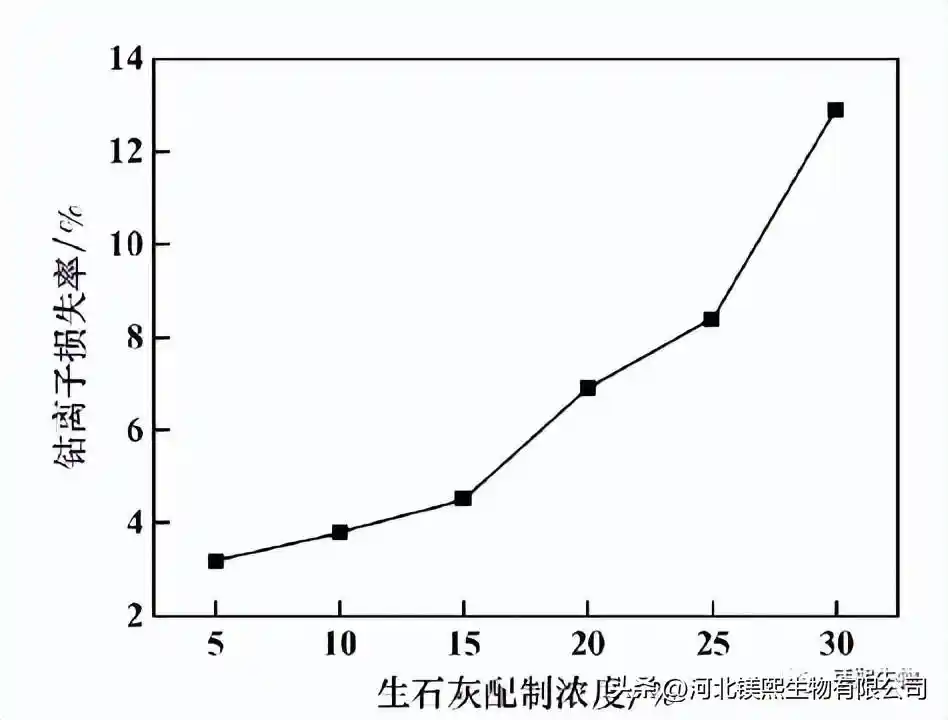
Figure 2 The effect of quicklime preparation concentration on the loss rate of cobalt ions
2.1.2 Influence of reaction time on impurity removal effect
The concentration of quicklime is 15%, the pH=4.0, and the potential is 420mV. The effect of reaction time on the impurity removal effect is shown in Figure 3. It can be seen from Figure 3 that, firstly, with the prolongation of the reaction time, the removal rate of impurity ions gradually increases. The removal rates of iron, copper, and manganese ions tended to be stable at 5h, 4h, and 6h, respectively. Considering comprehensively, it is advisable to choose 5 hours for the reaction time of impurity removal.
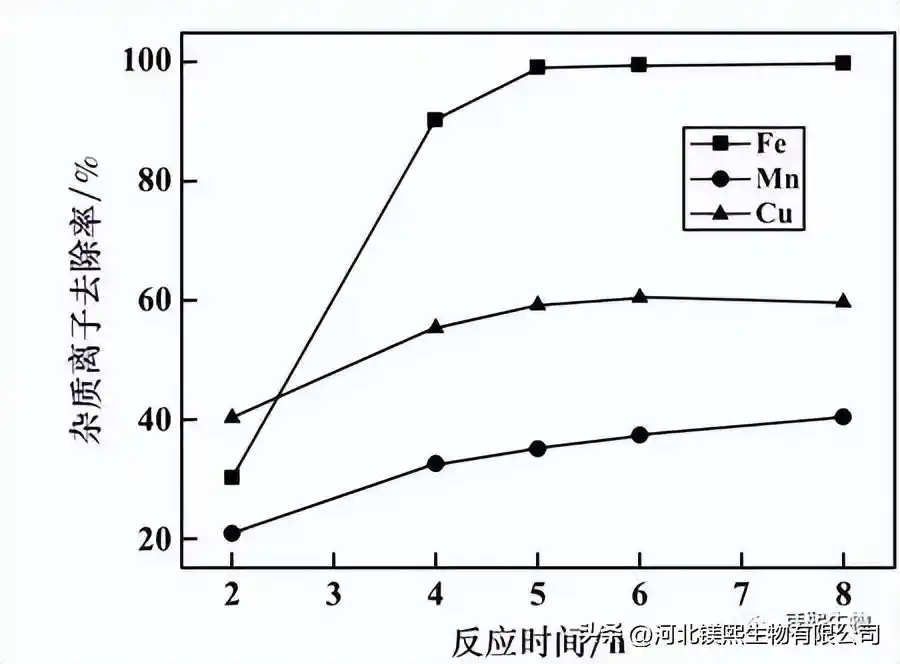
Figure 3 The influence of reaction time on the effect of impurity removal
2.1.3 Effect of reaction pH value on impurity removal effect
The influence of pH value on the removal rate of impurity ions is shown in Figure 4 when the concentration of quicklime is 15%, the reaction time is 5h, and the potential is 420mV. It can be seen from Figure 4 that the removal rate of impurity ions in the initial stage of the reaction increases with the increase of pH value. When the pH=3.5, the iron removal rate was 92.3%, and when the pH=4.0, the iron removal rate tended to be stable, maintaining above 99.1%, and continuing to increase the pH value had little effect on iron removal. When pH=4.5, the removal rate of copper ions reaches 60.4%, and when pH=5.5, the removal rate reaches 99.8%. When pH=5.0, the removal rate of manganese ions tended to be stable and maintained at about 41.5%. Excessively high pH value will cause greater loss of cobalt ions. In principle, under the condition of ensuring a good enough impurity removal effect, the lower the pH value, the better. Considering comprehensively, the pH value of impurity removal reaction should be 4.5.
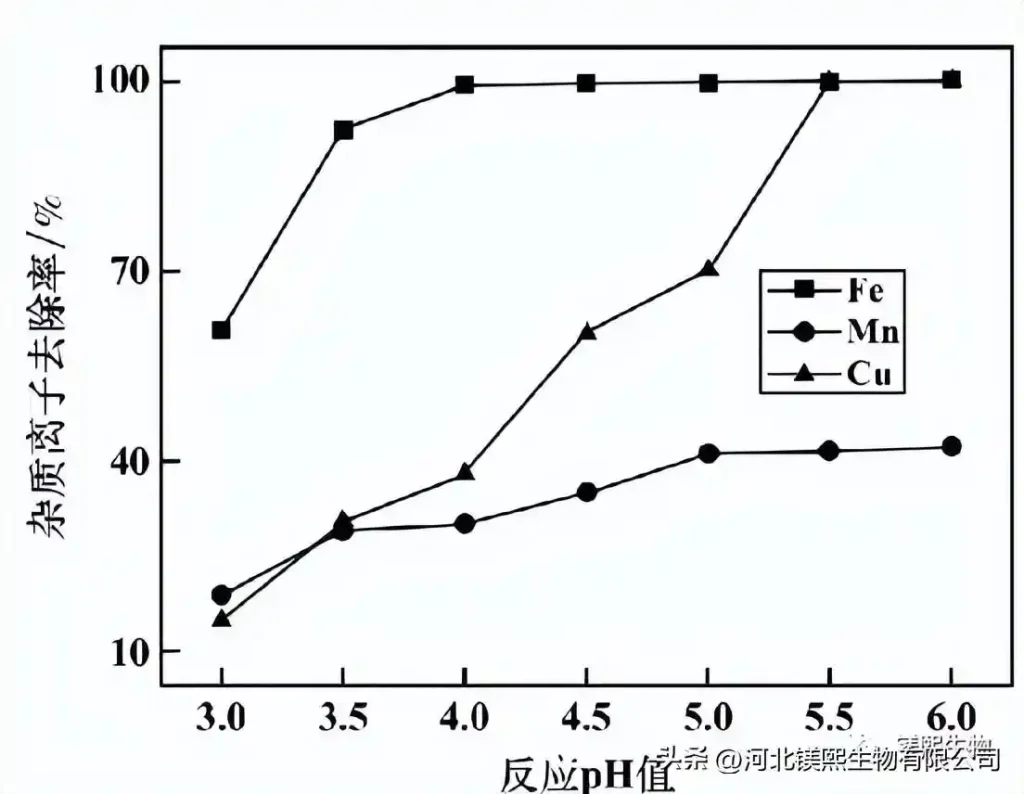
Figure 4 The influence of reaction pH value on the effect of impurity removal
2.1.4 Effect of reaction potential on impurity removal effect
The concentration of quicklime is 15%, the reaction time is 5h, and pH=4.5. The influence of potential on the impurity removal effect is shown in Figure 5. It can be seen from Figure 5 that the removal rate of impurity ions gradually increases with the increase of the reaction potential. When the potential rises above 420mV, the iron removal rate tends to be stable and reaches more than 99.2%. When the potential is above 450mV, the removal rate of manganese ions does not change significantly and remains at about 41%. Considering comprehensively, the impurity removal reaction potential is controlled at about 420mV.
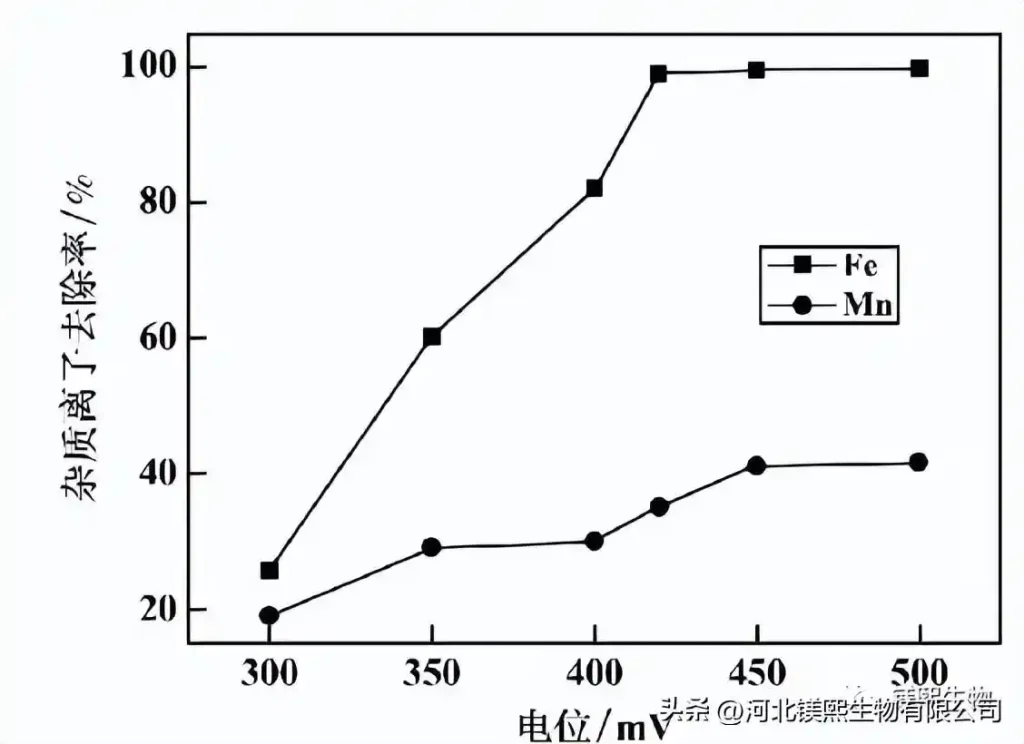
Figure 5 Effect of reaction potential on impurity removal effect
2.2 One-stage cobalt precipitation process conditions
2.2.1 Effect of pH value on the quality of cobalt hydroxide of the first stage of cobalt precipitation
The reaction time was 5 hours, and the amount of magnesium oxide added (tMgO/tCo) was 0.8. The effect of pH value on the quality of cobalt hydroxide in the first stage of cobalt precipitation was investigated. The results are shown in Figure 6.
It can be seen from Figure 6 that the grade of cobalt hydroxide Co increases with the increase of pH value at the beginning, reaches the maximum value (45.6%) at pH = 8, and then gradually decreases. This is because as the pH value gradually increases, the alkalinity in the solution becomes stronger and stronger, the amount of cobalt ion precipitation increases, and the proportion of cobalt ion in the product is also higher, but when the pH value increases to a certain level Finally, the precipitation of cobalt ions in the solution is complete, and other impurity ions also begin to precipitate in large quantities. At the same time, the magnesium oxide auxiliary materials added in large quantities are not completely consumed and also enter the cobalt hydroxide product, resulting in a decline in the cobalt grade in the cobalt hydroxide. However, the changing trend of magnesium impurity ions in the product is just the opposite. At the beginning, as the pH value gradually increased, a large amount of cobalt ions in the solution were precipitated, and the magnesium ions in the magnesium oxide slurry were basically completely consumed, and a small amount was transferred to the product. When the pH value reaches more than 8, the magnesium oxide in the solution is excessive, and a relatively large proportion of magnesium oxide enters the cobalt hydroxide product, thereby causing the magnesium impurity content in the product to gradually increase. Considering comprehensively, it is advisable to choose a pH value of about 8.0 for the first-stage cobalt precipitation reaction.
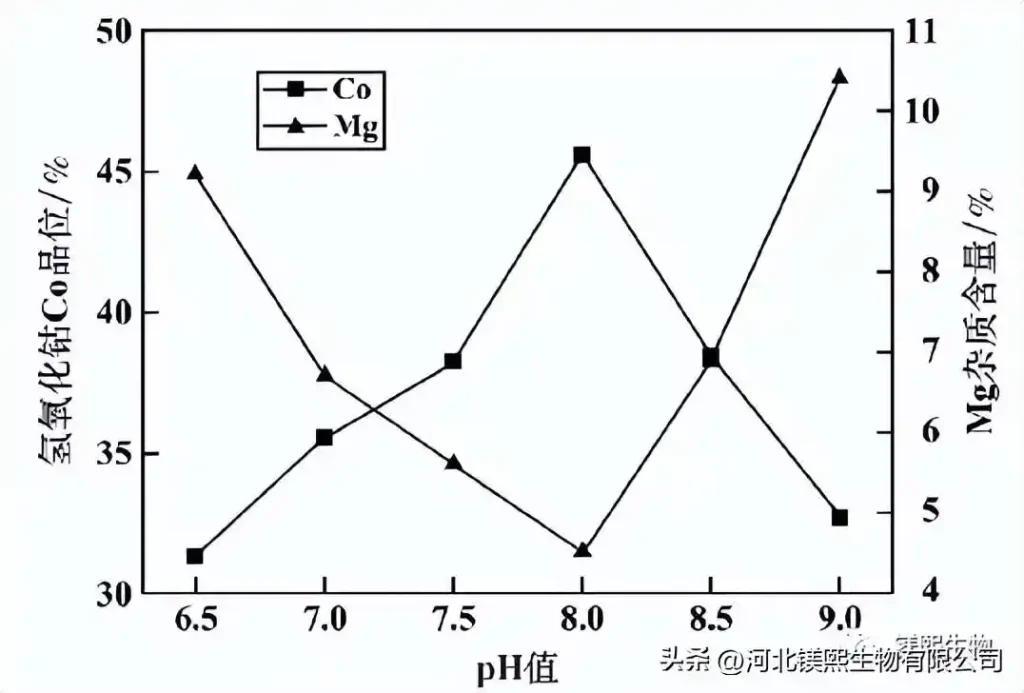
Fig. 6 Effect of pH value on the quality of cobalt hydroxide in the first stage
2.2.2 The effect of reaction time on the quality of cobalt hydroxide for a period of precipitated cobalt
The reaction pH=8.0, the addition amount of magnesium oxide (tMgO/tCo)0.8, and the effect of reaction time on the quality of cobalt hydroxide in one stage are shown in Figure 7. It can be seen from Figure 7 that the grade of cobalt hydroxide Co gradually increases with the prolongation of the reaction time, and tends to be stable after the reaction time reaches 6 h; the content of impurity magnesium gradually decreases with the prolongation of the reaction time, and tends to be stable after the reaction time reaches 8 h. This is mainly because as the reaction time prolongs, more and more cobalt ions in the solution are precipitated, and at the same time, the unutilized magnesium oxide is gradually dissociated and re-participated in the cobalt precipitation reaction, thereby gradually reducing the magnesium content in the product. Improve the product cobalt grade. Considering comprehensively, it is advisable to choose 6h as the reaction time for one stage of cobalt precipitation.
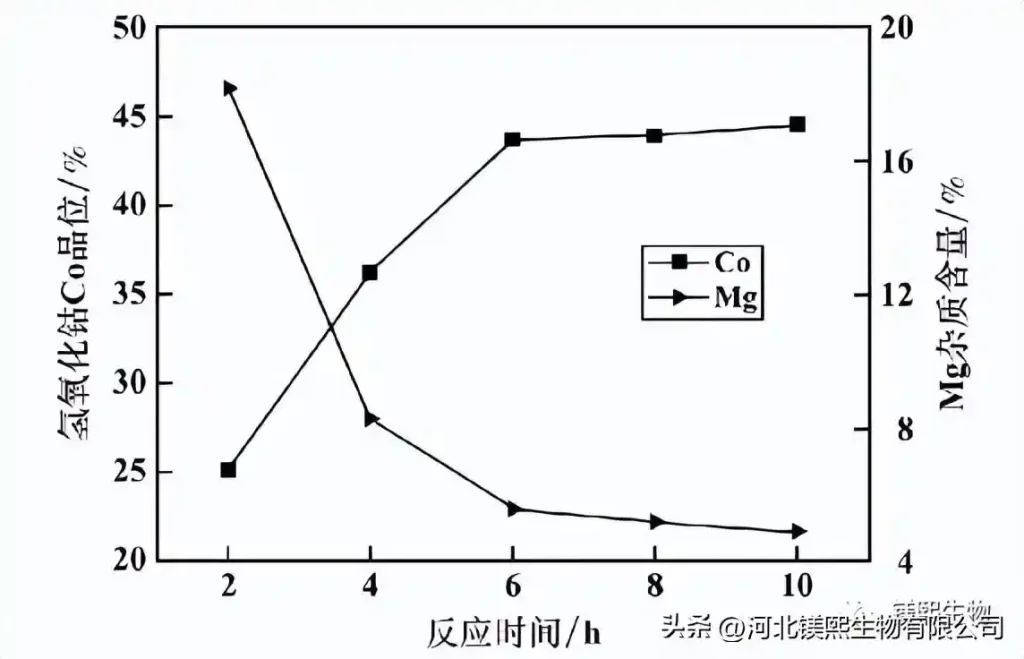
Figure 7 The influence of reaction time on the quality of one-stage cobalt-cobalt hydroxide
2.2.3 The effect of magnesium oxide addition on the quality of cobalt hydroxide in the first stage of cobalt precipitation
Reaction pH=8.0, reaction time 6h, the effect of magnesium oxide addition amount (tMgO/tCo) on the quality of cobalt hydroxide in the first stage is shown in Figure 8. It can be seen from Figure 8 that the grade of cobalt hydroxide Co increases gradually with the increase of the addition of magnesium oxide at first, but when the addition of magnesium oxide reaches 1.0, the grade of cobalt hydroxide Co reaches the maximum value, and then increases with the addition of magnesium oxide. increase and decrease. The magnesium impurity content first decreased gradually with the increase of magnesium oxide addition. When the magnesium oxide addition was 1.0, the magnesium impurity content was the lowest, and then began to gradually increase. This is mainly because when the amount of magnesia added is insufficient, a large amount of cobalt ions begin to precipitate out of the solution with the gradual addition of magnesia, and the added magnesia is replaced and consumed at the same time, and there is less residue in the precipitate, but when magnesia is added When the amount reaches more than 1.0, as the added amount continues to increase, the excess of magnesium oxide becomes more and more, and the magnesium oxide that cannot be completely consumed directly enters the cobalt hydroxide precipitation, resulting in lower and lower cobalt grades and lower magnesium impurity content. Higher and higher. Considering comprehensively, tMgO/tCo=1.0 is suitable for the first-stage cobalt precipitation reaction.
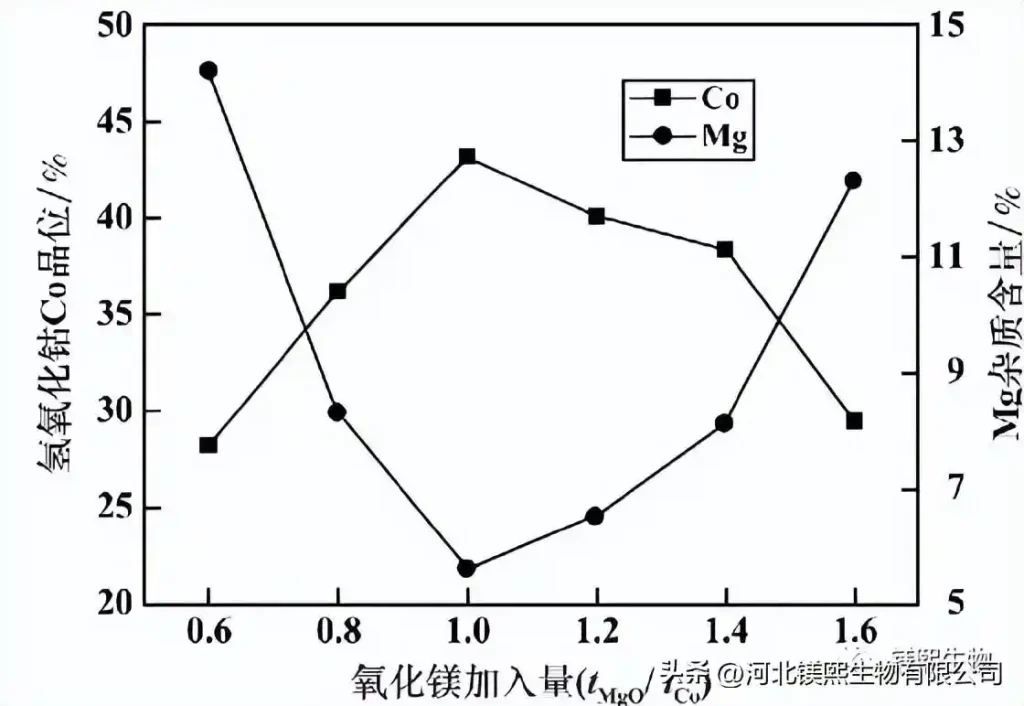
Figure 8 Effect of magnesium oxide addition on the quality of cobalt hydroxide in the first stage
2.3 Second stage cobalt precipitation process conditions
2.3.1 Effect of pH value on the quality of the second-stage cobalt sedimentation slag
The reaction time was 4 hours, and the effect of pH value on the cobalt quality of the second-stage cobalt precipitation slag was investigated, and the results are shown in Figure 9. It can be seen from Figure 9 that at pH=9.0, the Co content of the second-stage cobalt precipitation slag reaches 34.2%, and then as the pH value gradually increases, the cobalt content continues to decrease, while the magnesium oxide content increases sharply. This is because the raw material of the second-stage cobalt precipitation is the liquid after the first-stage cobalt precipitation, and the concentration of cobalt ions in the solution is generally low. In the second-stage cobalt precipitation process, the unprecipitated cobalt ions in the first-stage cobalt precipitation process must continue to precipitate, and the requirements must be higher. The pH value means that the magnesium oxide must be partially excessive, but if the pH value is too high, a large amount of impurity ions such as Mn and Mg will precipitate, which will lead to a sharp decrease in the Co content of the second-stage cobalt slag, and at the same time, the content of impurities such as magnesium will rapidly decrease. raised. Considering comprehensively, the pH value of the second-stage cobalt precipitation should be around 9.0.
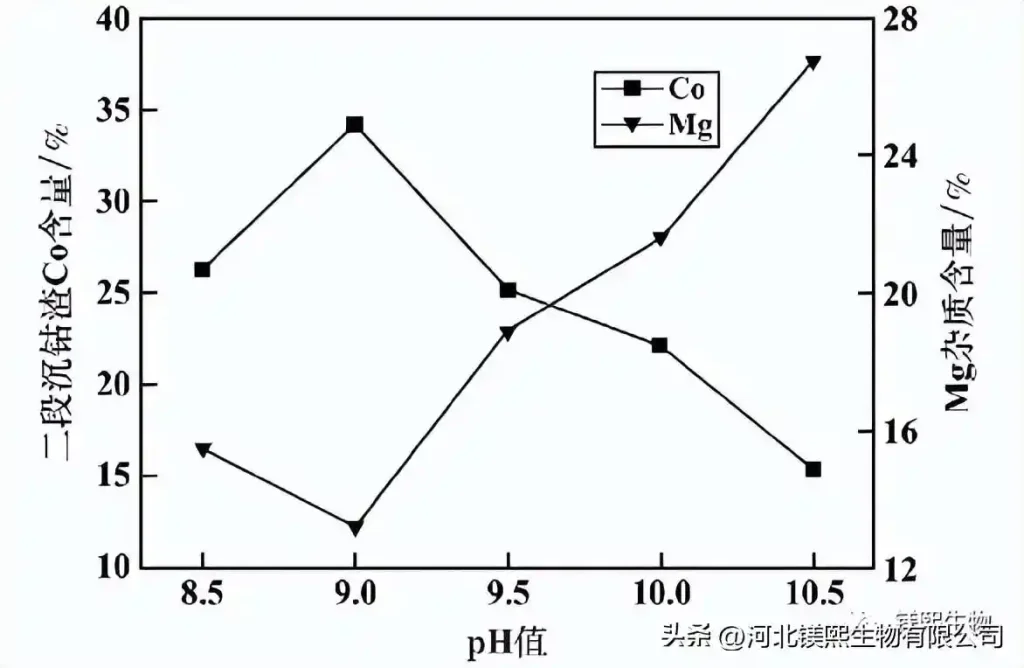
Figure 9 Effect of pH value on the quality of the second-stage cobalt sedimentation slag
2.3.2 Effect of reaction time on the quality of the second-stage cobalt deposit
The reaction pH=9.0, the effect of reaction time on the quality of the second-stage cobalt sedimentation slag is shown in Figure 10. It can be seen from Figure 10 that as the reaction time prolongs, the Co content in the second-stage cobalt deposit slag increases, while the Mg impurity content decreases, and the reaction time reaches more than 3 hours, and the Co content and Mg impurity content in the second-stage cobalt deposit slag tend to be stable. . Considering comprehensively, it is advisable to choose 3h as the reaction time of the second stage of Co precipitation.
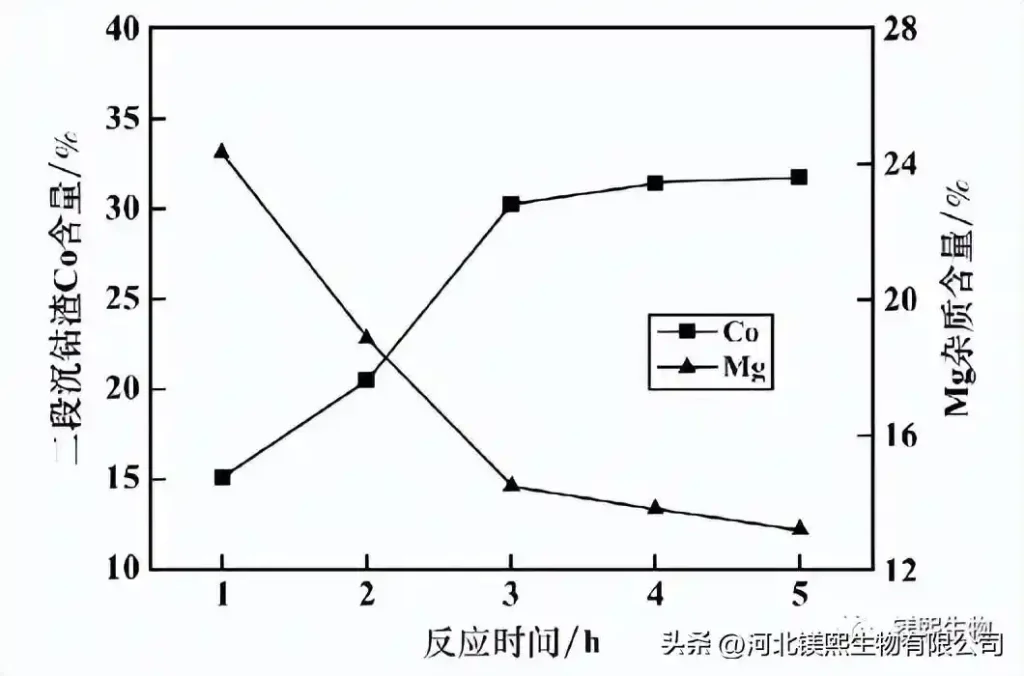
Figure 10 Effect of reaction time on the quality of the second-stage cobalt sedimentation slag
2.4 Precipitated cobalt products prepared under optimized process conditions
Through the above single factor experiment, the suitable process conditions for impurity removal are: quicklime concentration 15%, reaction time 5h, reaction pH=4.5, potential 420mV; the suitable process conditions for cobalt precipitation in the first stage are: reaction pH=8.0, reaction time 6h, oxidation The amount of magnesium added (tMgO/tCo) is 1.0; the suitable process conditions for the second-stage cobalt precipitation are: reaction time 3h, reaction pH=9.0; and all the second-stage cobalt precipitation residues are returned to the first-stage cobalt precipitation process as reaction seeds. A verification experiment was carried out under this optimized process condition, and the analysis results of the obtained cobalt hydroxide sample are shown in Table 2. It can be seen from Table 2 that the cobalt hydroxide Co grade reaches 45.60% under the optimized conditions of the first-stage cobalt precipitation; The Co content of the final product also reaches 39.65%, which fully meets the product sales requirements.
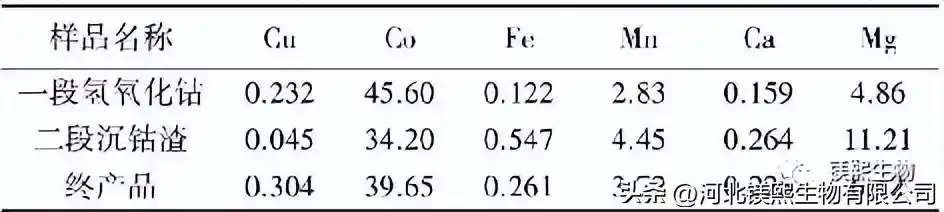
The main components (mass fraction)/% of the cobalt hydroxide sample prepared under the optimized process conditions in table 2
3 Conclusion
1) The optimized production process conditions for impurity removal reaction are: quicklime concentration 15%, reaction time 5h, reaction pH=4.5, potential 420mV, and the iron removal rate under these optimized conditions is 99.5%.
2) The optimized process conditions of the first-stage cobalt precipitation are: reaction pH=8.0, reaction time 6h, magnesium oxide addition (tMgO/tCo) 1.0, the cobalt hydroxide Co grade prepared under these optimized conditions reaches 45.6%.
3) The optimized process conditions of the second-stage cobalt precipitation are: reaction time 3h, reaction pH=9.0, the Co content of the second-stage cobalt precipitation slag obtained under these optimized conditions reaches 34.2%, and all the second-stage cobalt precipitation slag is returned to the first-stage cobalt precipitation Cobalt is used as the reaction seed crystal, and the Co content of the final cobalt salt product reaches 39.65%.
