Hebei Messi Biology Co., Ltd. stated that nanomagnesium oxide (MgO), as a new type of multifunctional inorganic material, has broad application prospects in many fields, especially in environmental purification and antibacterial aspects that are closely related to human survival and health. unique advantage. The incorporation of foreign elements into the crystal lattice of oxides will produce lattice defects such as vacancies, interstitial atoms, replacement atoms and dislocations, and the generation of these defects can promote the physical and chemical properties of the parent oxide to a certain extent. Based on this point, this paper selects Ti4+, Zn2+ and Li+, three metal ions of different valence states with similar ionic radii to Mg2+, as dopants to improve the adsorption and antibacterial properties of nano-magnesium oxide through doping.
First, a brief overview of the research progress of magnesium oxide in environmental purification and antibacterial aspects as well as the preparation methods and doping modification of nano-magnesium oxide are briefly summarized. Then the modified precipitation method or sol-gel method was used to prepare nano-magnesium oxide and doped nano-magnesia powder respectively, and the effects of the preparation process parameters and doping on the powder structure and morphology were studied. Finally, the effects of three different ion dopings on the adsorption and antibacterial properties of nano-magnesium oxide powder were studied, and on this basis, the mechanism of doping-promoting properties was preliminarily discussed.
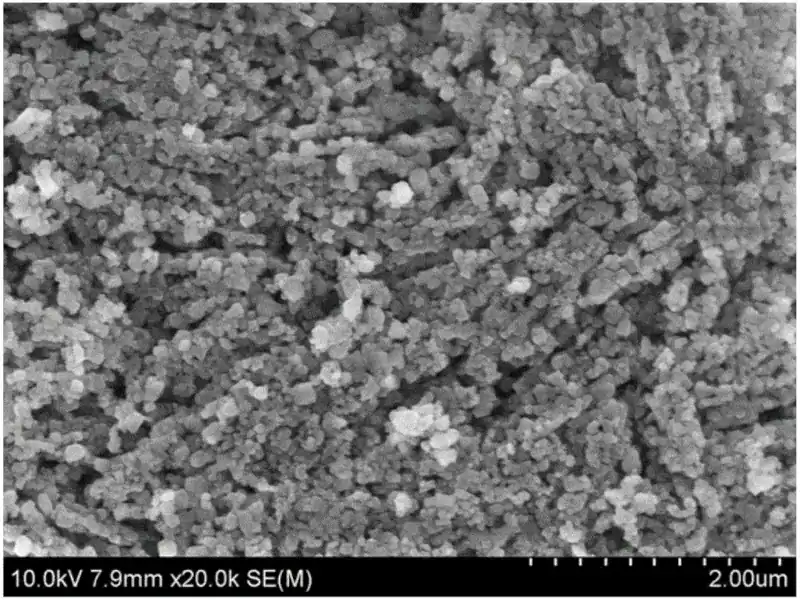
It mainly includes the following aspects: Using polyethylene glycol (PEG) as the dispersant, nanometer magnesium oxide powder was prepared by direct chemical precipitation. The results of the orthogonal experiment show that the reaction temperature has the greatest impact on the crystal grains, and the other influencing factors are PEG dosage, reaction time, ammonia dosage and Mg2+ concentration; in terms of yield, the ammonia dosage has the greatest impact, followed by PEG dosage and reaction time. The influence of reaction temperature is secondary, and the influence of Mg2+ concentration is the smallest. The preparation process conditions of nano-magnesium oxide were determined as follows: ammonia dosage 8mL, Mg2+ concentration 0.5mol/L, PEG dosage 8mL, reaction temperature 50℃, reaction time 1.5h, and calcination temperature 500℃. PEG affects the shape and size of powder particles. When the number of magnesium hydroxide nucleation is small, a flaky morphology is formed; as the number of nucleation increases, a “network”-like morphology of densely packed fine particles is formed.
Using glacial acetic acid as a modifier can not only avoid the phenomenon of uneven precipitation caused by excessive local precipitant concentration in the direct precipitation method, but also promote the oriented growth of the precursor magnesium hydroxide crystal on the (001) plane, reducing the amount of precipitation caused by hydrogen. The thermal decomposition temperature of the oxide to the oxide is conducive to the crystallization of the oxide. For titanium-doped nanomagnesium oxide prepared by direct precipitation method, the particle size of the obtained powder increases significantly with the increase of titanium doping amount, and the hard agglomeration is serious; while the particle size of the sample prepared by modified precipitation method increases with the increase of titanium doping amount. Reduced, with less agglomeration and good dispersion. After the prepared precursor was calcined at 500°C, all samples contained only magnesium oxide phase. However, after calcining at 800°C, samples containing titanium doped with more than 1 mol% had titanium-containing impurity phases precipitated. Therefore, it can be confirmed that titanium is present in magnesium oxide. The solid solubility in the crystal lattice is about 1 mol%.
As the amount of titanium added increases, the crystallization properties of magnesium oxide decrease and the crystal grains become smaller. In the preparation of zinc-doped nanomagnesium oxide by the modified precipitation method, the study found that the increase in zinc content had basically no effect on the intensity of the diffraction peak and the size of the crystal grains and particles. Compared with samples prepared without adding glacial acetic acid, the powder prepared using glacial acetic acid has larger grain size and better crystallization properties. It shows that glacial acetic acid promotes the transformation from hydroxide to oxide during the preparation process, which is conducive to the crystallization of the product. This is consistent with the conclusion in the preparation of nano-magnesium oxide by modified precipitation method. Nano-magnesium oxide and lithium-doped nano-magnesium oxide were prepared using a cheap inorganic sol-gel method. In the process of preparing nano-magnesium oxide, the amount of solvent, reaction temperature and citric acid dosage have varying degrees of impact on gel time and crystal grains. The heat treatment of xerogel adopts step-by-step pretreatment at low temperature for a period of time, and then calcining at 600°C for 2 hours to produce white high-purity nanomagnesium oxide.
The thermal decomposition temperature of xerogel shifts toward high temperature after doping with lithium. After calcination at 600°C, there is only magnesium oxide phase in the 0.5% lithium and 1% lithium samples, while there are impurity phases in the samples with high lithium content. As the amount of lithium doped increases, the intensity of the diffraction peak becomes stronger, the grain and particle size become larger, and the morphology changes from granular to flake-like. After calcination at 800°C, impurities exist in all lithium-doped samples, and the diffraction peaks are sharper and stronger.
Methyl orange and Escherichia coli were used as models to study the adsorption and antibacterial properties of the prepared powder. As the adsorption performance of nano-magnesium oxide becomes smaller, the adsorption amount of methyl orange becomes larger and the adsorption rate becomes faster. As the treatment temperature increases, the adsorption amount when reaching adsorption equilibrium increases slightly, but the adsorption rate slows down.
The adsorption performance of titanium-doped MgO on methyl orange is better than that of pure magnesium oxide, among which the 1% titanium sample has the best adsorption performance; the adsorption performance of zinc-doped magnesium oxide begins to weaken as the zinc content increases, but when When the zinc doping amount reaches 15 mol%, although the adsorption rate slows down, the adsorption amount at equilibrium increases instead; the adsorption performance of lithium-doped MgO gradually weakens as the lithium doping amount increases. Experiments on Escherichia coli show that the antibacterial properties of titanium-doped MgO are worse than those of pure MgO; in zinc-doped MgO samples, 5% zinc and 10% zinc have good bactericidal properties, while 1% zinc has almost no antibacterial effect; 1% lithium The antibacterial performance of 5% lithium and 5% lithium is basically equivalent to that of pure magnesium oxide, while the antibacterial effect of 10% lithium is slightly better. The sterilization rate of lithium-doped MgO is over 99%, showing excellent sterilization performance.

