Principle
The brine ammonia reverse precipitation method utilizes the chemical reaction between brine (containing magnesium chloride) and ammonia to generate magnesium hydroxide precipitation, and by controlling the reaction conditions, the product of magnesium hydroxide with hexagonal flake shape is obtained.
Process flow
Pre-treatment of raw materials: brine is clarified, concentrated and decontaminated to obtain qualified concentrated brine. Ammonia is generally used industrial grade ammonia.
Pre-precipitation: Concentrated brine and ammonia are mixed in the reactor, and ammonia is slowly passed in to generate the initial precipitation of magnesium hydroxide.
Reverse precipitation: In the system of magnesium hydroxide primary precipitation, continue to pass a large amount of ammonia gas, dissolve the primary precipitation into amino complexes, and then by adjusting the temperature, stirring speed and other conditions, so that it will be re-precipitated to form hexagonal flake morphology of magnesium hydroxide.
Filtering and промывка: After the reaction, the precipitate is filtered, промывка and dried to get the finished product of hexagonal flake magnesium hydroxide.
Reaction mechanism
In the brine ammonia reverse precipitation method, the reaction is mainly divided into the following three stages:
Pre-precipitation stage: MgCl2 + 2NH3 + H2O → Mg(OH)2 + 2NH4Cl
Dissolution stage: Mg(OH)2 + 6NH3 → [Mg(NH3)6]2+ + 2OH-
Reverse precipitation stage: [Mg(NH3)6]2+ + 2OH- + 2NH4Cl → Mg(OH)2 + 8NH3
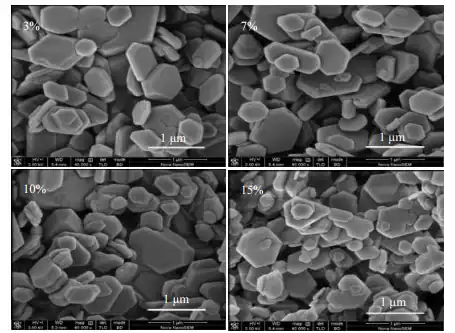
Factors affecting the morphology of hexagonal flakes
Ammonia excess coefficient: the larger the ammonia excess coefficient, the more obvious the hexagonal flake morphology.
Temperature: Higher temperature is favorable to the formation of hexagonal flake shape.
Stirring speed: moderate stirring speed is favorable to provide enough nuclei to promote the growth of hexagonal flake crystals.
Other factors: impurity ions, surfactants, etc. also affect the shape of hexagonal flake.
Advantages
The prepared hexagonal flake magnesium hydroxide has high activity, adsorption capacity and flame retardancy.
The process is controllable and the product quality is stable.
Abundant source of raw materials, low cost.
Application



Hexagonal flake magnesium hydroxide is widely used in paper making, rubber, plastic, medicine, environmental protection and other fields, such as:
Fillers and coatings in paper industry
Reinforcing agent and flame retardant in rubber industry
Antacid and laxative in pharmaceutical industry
Adsorbent and flocculant in environmental protection industry.
