Hebei Messi Biology Co., Ltd. stated that the adsorption performance of magnesium oxide materials is closely related to its specific surface area. Generally, materials with higher specific surface areas have better adsorption effects. Rod-shaped ones have the best adsorption performance for Congo red among three different shapes of magnesium oxide. With the most superior morphology, sodium silicate is the additive with the best performance among the six inorganic salts.
We have proposed a synthesis method for rod-shaped magnesium oxide with ultra-high adsorption capacity for Congo red in aqueous solution, with an adsorption capacity of up to 3236mg/L. This rod-shaped material is produced by adding a small amount of silicic acid to the reaction system of magnesium nitrate and sodium carbonate. Prepared from sodium. During the reaction process, experimental parameters such as the amount of sodium silicate added, stirring time and roasting temperature affect the alkalinity and alkali content of the surface of the material, so the adsorption performance of the prepared magnesium oxide is different, but it is different from the material’s The specific surface area is almost irrelevant.
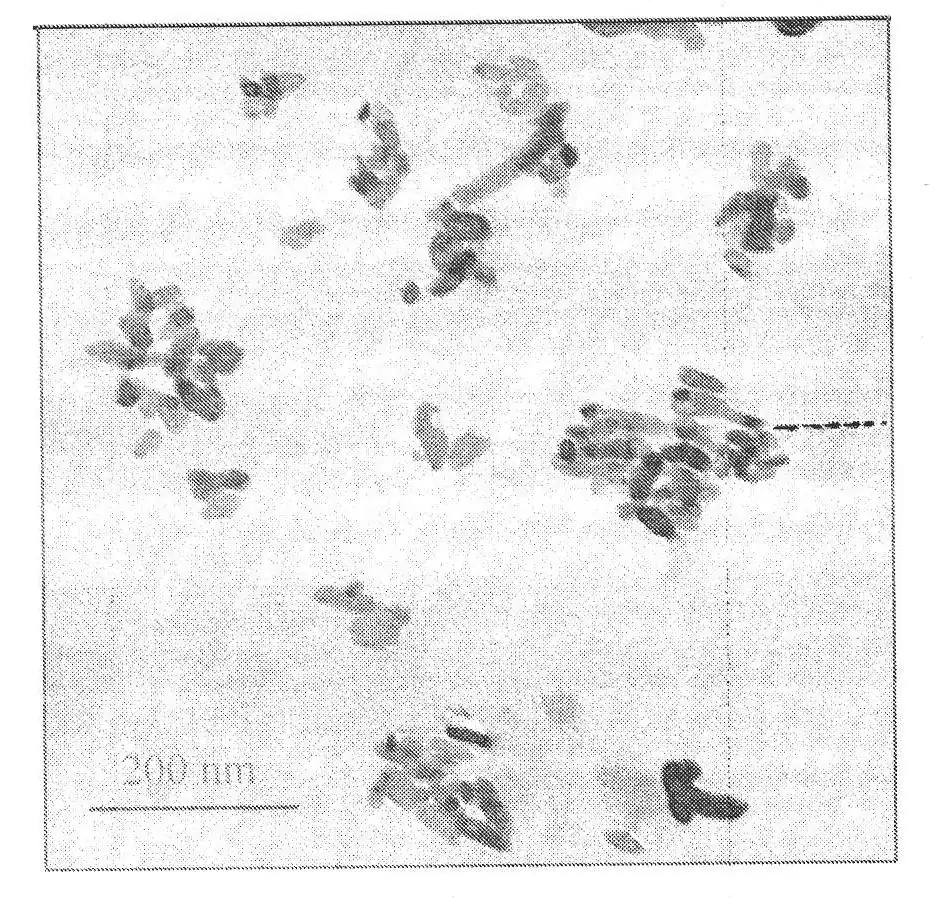
For rod-shaped materials, in addition to sodium silicate, adding a small amount of sodium phosphate, sodium tripolyphosphate and multi-stage sodium phosphate to the carbonate precipitant and magnesium source reaction system will also improve the adsorption performance of the final product, but fluorination The addition of sodium and sodium oxalate is not conducive to improving the adsorption performance of the prepared materials. In addition to rod-shaped magnesium oxide, in the synthesis of spherical and trapezoidal magnesium oxide materials, adding a small amount of sodium silicate to the precipitant solution will also improve the adsorption performance of the synthesized materials.
By systematically studying the adsorption process of Congo red on the surface of magnesium oxide, it was found that the adsorption process is more consistent with the pseudo-second-order rate equation and the Langmuir adsorption model. By reviewing the literature on the adsorption of Congo red by magnesium oxide, it can be seen that the adsorption capacity of this rod-shaped material is the largest reported amount of Congo red adsorption by magnesium oxide so far. Compared with the synthesis routes of other morphological magnesium oxide adsorbents, the synthesis method used in this article is simpler and controllable, which is beneficial to its industrial production and efficient treatment of Congo red waste liquid.

